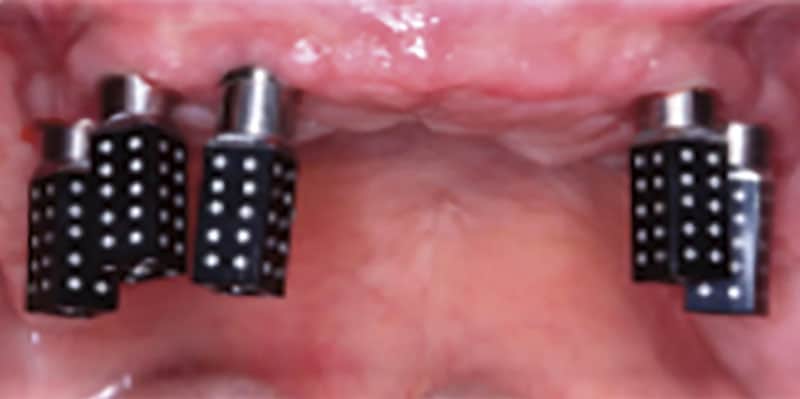
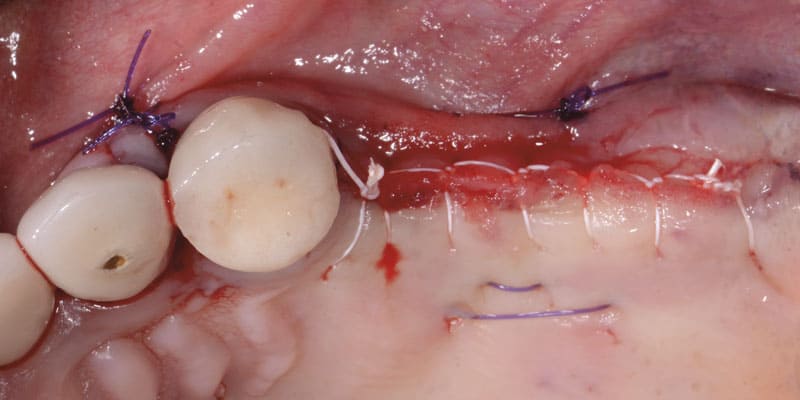
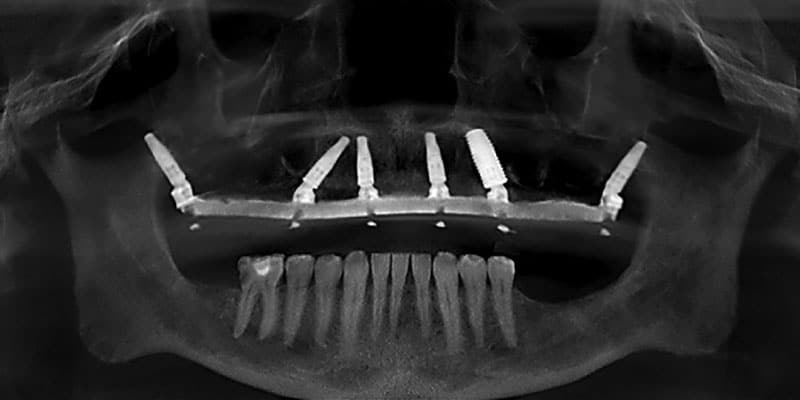
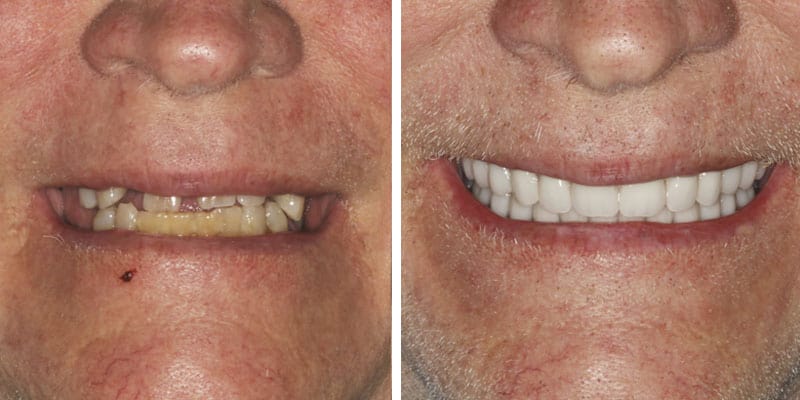
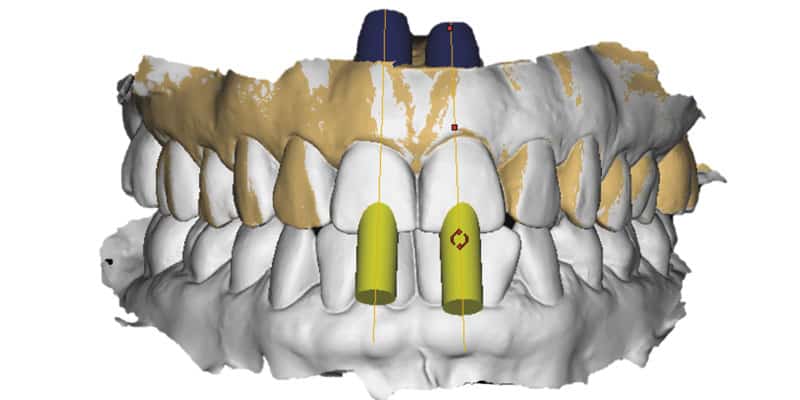
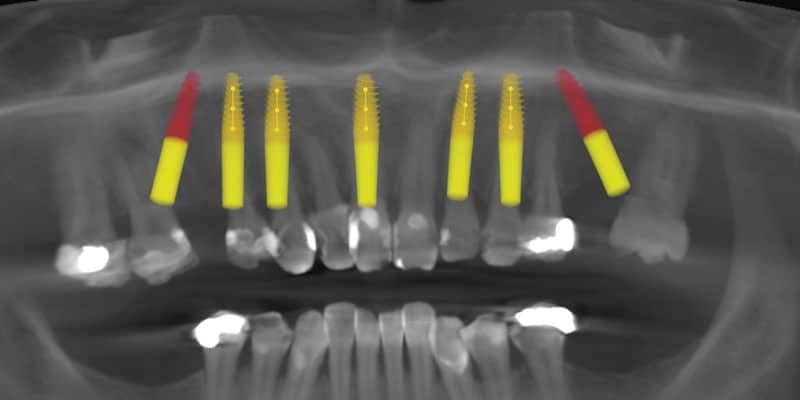
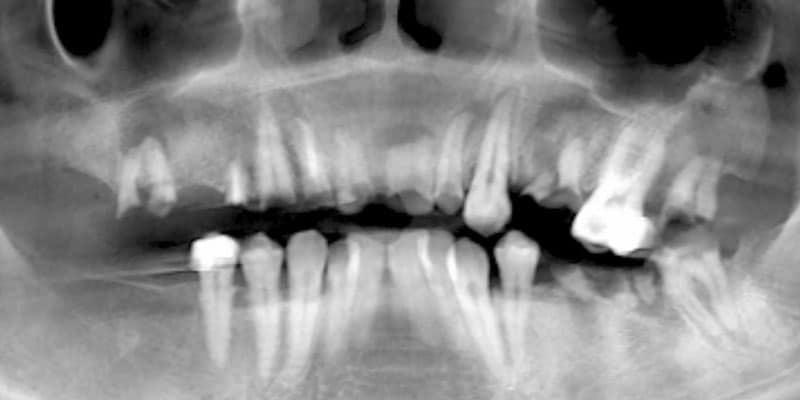
FOLLOW US ONLINE
USEFUL LINKS
SUBSCRIBE TODAY
Implant Practice US is a leading dental journal and publication for dental implantology continuing education, oral implantology case studies, and more. Subscribe to Implant Practice US today!
ONLINE DENTAL CE
Earn dental continuing education credits as an Implant Practice US subscriber. Log in for online dental CE credits now!
Other Dental Publications
FOLLOW US ONLINE
USEFUL LINKS
SUBSCRIBE TODAY
Implant Practice US is a leading dental journal and publication for dental implantology continuing education, oral implantology case studies, and more. Subscribe to Implant Practice US today!
ONLINE DENTAL CE
Earn dental continuing education credits as an Implant Practice US subscriber. Log in for online dental CE credits now!
Other Dental Publications
Copyright © 2024 Orthodontic Practice US - Dental Journal and Online Dental CE | MedMark LLC
15720 North Greenway Hayden Loop, Suite #9 Scottsdale, AZ 85260 | All rights Reserved | Privacy Policy | Terms & Conditions